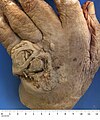
Cutaneous squamous-cell carcinoma
| Cutaneous squamous-cell carcinoma | |
|---|---|
| Other names | Squamous-cell carcinoma of the skin, squamous-cell skin cancer, epidermoid carcinoma, squamous-cell epithelioma of the skin |
 | |
| Cutaneous squamous-cell carcinoma tends to arise from actinic keratoses (premalignant lesions); surface is usually scaly and often ulcerates (as shown here). | |
| Specialty | Dermatology, plastic surgery, otorhinolaryngology |
| Symptoms | Hard lump with a scaly top or ulceration.[1] |
| Risk factors | Ultraviolet radiation, actinic keratosis, tobacco smoking, lighter skin, arsenic exposure, radiotherapy, poor immune system function, HPV infection[2] |
| Diagnostic method | Tissue biopsy[2][3] |
| Differential diagnosis | Keratoacanthoma, actinic keratosis, melanoma, warts, basal cell cancer[4] |
| Prevention | Decreased UV radiation exposure, sunscreen[5][6] |
| Treatment | Surgical removal, radiotherapy, chemotherapy, immunotherapy[2][7] |
| Prognosis | Usually good[5] |
| Frequency | 2.2 million (2015)[8] |
| Deaths | 51,900 (2015)[9] |
Cutaneous squamous-cell carcinoma (cSCC), also known as squamous-cell carcinoma of the skin or squamous-cell skin cancer, is one of the three principal types of skin cancer, alongside basal-cell carcinoma and melanoma.[10] cSCC typically presents as a hard lump with a scaly surface, though it may also present as an ulcer.[1] Onset and development often occurs over several months.[4]
Compared to basal cell carcinoma, cSCC is more likely to spread to distant areas.[11] When confined to the epidermis, the outermost layer of the skin, the pre-invasive or in situ form of cSCC is termed Bowen's disease.[12][13]
The most significant risk factor for cSCC is extensive lifetime exposure to ultraviolet radiation from sunlight.[2] Additional risk factors include prior scars, chronic wounds, actinic keratosis, lighter skin susceptible to sunburn, Bowen's disease, exposure to arsenic, radiation therapy, tobacco smoking, poor immune system function, previous basal cell carcinoma, and HPV infection.[2][14][15] The risk associated with UV radiation correlates with cumulative exposure rather than early-life exposure.[16] Tanning beds have emerged as a significant source of UV radiation.
Genetic predispositions, such as xeroderma pigmentosum[17] and certain forms of epidermolysis bullosa,[18] also increase susceptibility to cSCC. The condition originates from squamous cells located in the skin's upper layers.[19] Diagnosis typically relies on skin examination, and is confirmed through skin biopsy.[2][3]
Research, both in vivo and in vitro, indicates a crucial role for the upregulation of FGFR2, part of the fibroblast growth factor receptor immunoglobin family, in cSCC cell progression.[20] Mutations in the TPL2 gene leads to overexpression of FGFR2, which activates the mTORC1 and AKT pathways in primary and metastatic cSCC cell lines. Utilization of a "pan FGFR inhibitor" has shown to reduce cell migration and proliferation in cSCC in vitro studies.[20]
Preventive measures against cSCC include minimizing exposure to ultraviolet radiation and the use of sunscreen.[5][6] Surgical removal is the typical treatment method,[2] employing simple excision for minor cases or Mohs surgery for more extensive instances.[2] Other options include cryotherapy and radiation therapy.[7] For cases with distant metastasis, chemotherapy or biologic therapy may be employed.[7]
As of 2015, approximately 2.2 million individuals globally were living with cSCC at any given time,[8] constituting about 20% of all skin cancer cases.[21] In the United States, approximately 12% of males and 7% of females are diagnosed with cSCC at some point in their lives.[2] While prognosis remains favorable in the absence of metastasis, upon distant spread the five-year survival rate is markedly reduced to ~34%.[4][5] In 2015, global deaths attributed to cSCC numbered around 52,000.[9] The average age at diagnosis is approximately 66 years.[4] Following successful treatment of an initial cSCC lesion, there is a substantial risk of developing subsequent lesions.[2]
Signs and symptoms
[edit]
SCC of the skin begins as a small nodule and as it enlarges the center becomes necrotic and sloughs and the nodule turns into an ulcer, and generally are developed from an actinic keratosis. Once keratinocytes begin to grow uncontrollably, they have the potential to become cancerous and produce cutaneous squamous-cell carcinoma.[22]
- The lesion caused by cSCC is often asymptomatic
- Ulcer or reddish skin plaque that is slow growing
- Intermittent bleeding from the tumor, especially on the lip
- The clinical appearance is highly variable
- Usually the tumor presents as an ulcerated lesion with hard, raised edges
- The tumor may be in the form of a hard plaque or a papule, often with an opalescent quality, with tiny blood vessels
- The tumor can lie below the level of the surrounding skin, and eventually ulcerates and invades the underlying tissue
- The tumor commonly presents on sun-exposed areas (e.g. back of the hand, scalp, lip, and superior surface of pinna)
- On the lip, the tumor forms a small ulcer, which fails to heal and bleeds intermittently
- Evidence of chronic skin photodamage, as in multiple actinic keratoses (solar keratoses)
- The tumor grows relatively slowly
Spread
[edit]- Unlike basal-cell carcinoma (BCC), squamous-cell carcinoma (SCC) has a higher risk of metastasis.
- Risk of metastasis is higher clinically in SCC arising in scars, on the lower lips, ears, or mucosa, and occurring in immunosuppressed and solid organ transplant patients. Risk of metastasis is also higher in SCC that are > 2 cm in diameter, growth into the fat layer and along nerves, presence of lymphovascular invasion, poorly differentiated cell architecture on histology, or thickness greater than 6 mm.[23][24][25]
Causes
[edit]Cutaneous squamous-cell carcinoma is the second-most common cancer of the skin (after basal-cell carcinoma, but more common than melanoma). It usually occurs in areas exposed to the sun. Sunlight exposure and immunosuppression are risk factors for SCC of the skin, with chronic sun exposure being the strongest environmental risk factor.[26] There is a risk of metastasis starting more than 10 years[citation needed] after diagnosable appearance of squamous-cell carcinoma, but the risk is low,[specify] though much[specify] higher than with basal-cell carcinoma. Squamous-cell cancers of the lip and ears have high rates of local recurrence and distant metastasis.[27] In a recent study, it has also been shown that the deletion or severe down-regulation of a gene titled Tpl2 (tumor progression locus 2) may be involved in the progression of normal keratinocytes into becoming squamous-cell carcinoma.[28]
cSCC represents about 20% of the non-melanoma skin cancers; 80-90% of cSCCs with metastatic potential are located on the head and neck.[29]
Tobacco smoking also increases the risk for cutaneous squamous-cell carcinoma.[14][30]
The vast majority of cSCC cases are located on exposed skin, and are often the result of ultraviolet exposure. cSCC usually occurs on portions of the body commonly exposed to the sun; the face, ears, neck, hands, or arms. The primary sign is a growing bump that may have a rough, scaly surface, and flat, reddish patches. Unlike basal-cell carcinoma, cSCC carries a higher risk of metastasis than does basal-cell carcinoma, and may spread to the regional lymph nodes,[31]
Erythroplasia of Queyrat (SCC in situ of the glans or prepuce in males,[32] M[33]: 733 [34]: 656 [35] or the vulva in females.[36]) may be induced by human papilloma virus.[37] It is reported to occur in the corneoscleral limbus.[38] Erythroplasia of Queyrat may also occur on the anal mucosa or the oral mucosa.[39]
Genetically, cSCC tumors harbor high frequencies of NOTCH and p53 mutations as well as less frequent alterations in histone acetyltransferase EP300, subunit of the SWI/SNF chromatin remodeling complex PBRM1, DNA-repair deubiquitinase USP28, and NF-κB signaling regulator CHUK.[40]
A significant proportion of cSCC and its precursor lesions carry UV-induced p53 mutations. In fact, these mutations are present in up to 90% of cSCC cases. The detection of p53 mutations in precursor lesions indicates that this could be an early event in the development of squamous cell carcinoma.[41]
Immunosuppression
[edit]People who have received solid organ transplants are at a significantly increased risk of developing squamous-cell carcinoma due to the use of chronic immunosuppressive medication.[42] While the risk of developing all skin cancers increases with these medications, this effect is particularly severe for cSCC, with hazard ratios as high as 250 being reported, versus 40 for basal cell carcinoma.[43] The incidence of cSCC development increases with time posttransplant.[44] Heart and lung transplant recipients are at the highest risk of developing cSCC due to more intensive immunosuppressive medications used.[45]
Cutaneous squamous-cell carcinoma in individuals on immunotherapy or who have lymphoproliferative disorders (e.g. leukemia) tend to be much more aggressive, regardless of their location.[46] The risk of cSCC, and non-melanoma skin cancers generally, varies with the immunosuppressive drug regimen chosen. The risk is greatest with calcineurin inhibitors like cyclosporine and tacrolimus, and least with mTOR inhibitors, such as sirolimus and everolimus. The antimetabolites azathioprine and mycophenolic acid have an intermediate risk profile.[47]
Diagnosis
[edit]Diagnosis is confirmed via skin biopsy of the tissue or tissues suspected to be affected by SCC. The pathological appearance of a squamous-cell cancer varies with the depth of the biopsy. For that reason, a biopsy including the subcutaneous tissue and basilar epithelium, to the surface is necessary for correct diagnosis. The performance of a shave biopsy (see skin biopsy) might not acquire enough information for a diagnosis. An inadequate biopsy might be read as actinic keratosis with follicular involvement. A deeper biopsy down to the dermis or subcutaneous tissue might reveal the true cancer. An excision biopsy is ideal, but not practical in most cases. An incisional or punch biopsy is preferred. A shave biopsy is least ideal, especially if only the superficial portion is acquired.[citation needed]
Histological characteristics
[edit]Histopathologically, the epidermis in cSCC in situ (Bowen's disease) will show hyperkeratosis and parakeratosis. There will also be marked acanthosis with elongation and thickening of the rete ridges. These changes will overly keratinocytic cells which are often highly atypical and may in fact have a more unusual appearance than invasive cSCC. The atypia spans the full thickness of the epidermis, with the keratinocytes demonstrating intense mitotic activity, pleomorphism, and greatly enlarged nuclei. They will also show a loss of maturity and polarity, giving the epidermis a disordered or "windblown" appearance.[citation needed]
Two types of multinucleated cells may be seen: the first will present as a multinucleated giant cell, and the second will appear as a dyskeratotic cell engulfed in the cytoplasm of a keratinocyte. Occasionally, cells of the upper epidermis will undergo vacuolization, demonstrating an abundant and strongly eosinophilic cytoplasm. There may be a mild to moderate lymphohistiocytic infiltrate detected in the upper dermis.[12]
-
Histopathology of squamous-cell carcinoma in situ (black arrow), compared to normal skin, showing marked atypia.
-
Squamous-cell carcinoma in situ, showing prominent dyskeratosis and aberrant mitoses at all levels of the epidermis, along with marked parakeratosis.[12]
In situ disease
[edit]
Bowen's disease is essentially equivalent to and used interchangeably with cSCC in situ, when not having invaded through the basement membrane.[12] Depending on source, it is classified as precancerous[13] or cSCC in situ (technically cancerous but non-invasive).[48][49] In cSCC in situ (Bowen's disease), atypical squamous cells proliferate through the whole thickness of the epidermis.[12] The entire tumor is confined to the epidermis and does not invade into the dermis.[12] The cells are often highly atypical under the microscope, and may in fact look more unusual than the cells of some invasive squamous-cell carcinomas.[12]
-
cSCC in situ, high magnification, demonstrating an intact basement membrane.[12]
-
cSCC in situ
-
cSCC in situ
-
cSCC in situ
-
cSCC in situ
Erythroplasia of Queyrat is a particular type of Bowen's disease that can arise on the glans or prepuce in males,[32][33]: 733 [34]: 656 [35] and the vulva in females.[36] It mainly occurs in uncircumcised males,[36][50] over the age of 40.[39]
Invasive disease
[edit]In invasive cSCC, tumor cells infiltrate through the basement membrane. The infiltrate can be somewhat difficult to detect in the early stages of invasion: however, additional indicators such as full thickness epidermal atypia and the involvement of hair follicles can be used to facilitate the diagnosis. Later stages of invasion are characterized by the formation of nests of atypical tumor cells in the dermis, often with a corresponding inflammatory infiltrate.[12]
-
Gross slice of squamous-cell carcinoma of the skin
-
Superficially invasive cutaneous squamous-cell carcinoma. These lesions often do not show the marked pleomorphism and atypical nuclei of cSCC in situ, but manifest early keratinocyte invasion of the dermis.[12]
-
High magnification demonstrates the pleomorphism of the invading keratinocytes[12]
-
Invasive nests with characteristic large celled centers. Ulceration (at left) is common in invasive cSCC.
Degree of differentiation
[edit]-
Well-differentiated (yet invasive) cSCC, showing prominent keratinization. It may form pearl-like structures where dermal nests of keratinocytes attempt to mature in a layered fashion. Well-differentiated cSCC has slightly enlarged hyperchromatic nuclei with abundant amounts of cytoplasm. Intercellular bridges will frequently be visible.[12]
-
Moderately differentiated lesions of invasive cSCC show much less organization and maturation with significantly less keratin formation.[12]
-
Poorly differentiated, where attempts at keratinization are often no longer evident. This is a clear-cell squamous-cell carcinoma. The dysplastic cells infiltrated cords through the dermis. Poorly differentiated cSCC has greatly enlarged pleomorphic nuclei showing a high degree of atypia and frequent mitoses.[12]
-
Poorly differentiated clear-cell squamous-cell carcinoma. For this type of cSCC, immunostains will likely be required to classify it unless other areas of the tumor show obvious squamous-cell features such as seen here (arrow).
Prevention
[edit]Appropriate sun-protective clothing, use of broad-spectrum (UVA/UVB) sunscreen with at least SPF 50, and avoidance of intense sun exposure may prevent skin cancer.[51] A 2016 review of sunscreen for preventing cutaneous squamous-cell carcinoma found insufficient evidence to demonstrate whether it was effective.[52]
Management
[edit]Most cutaneous squamous-cell carcinomas are removed with surgery. A few selected cases are treated with topical medication. Surgical excision with a free margin of healthy tissue is a frequent treatment modality. Radiotherapy, given as external beam radiotherapy or as brachytherapy (internal radiotherapy), can also be used to treat cSCC. There is little evidence comparing the effectiveness of different treatments for non-metastatic cSCC.[53]
Mohs surgery is frequently utilized; considered the treatment of choice for squamous-cell carcinoma of the skin, physicians have also utilized the method for the treatment of squamous-cell carcinoma of the mouth, throat, and neck.[54] An equivalent method of the CCPDMA standards can be utilized by a pathologist in the absence of a Mohs-trained physician. Radiation therapy is often used afterward in high risk cancer or patient types.[55] Radiation or radiotherapy can also be a standalone option in treating cSCC.
As a non-invasive option brachytherapy serves a painless possibility to treat in particular but not only difficult to operate areas like the earlobes or genitals. An example of this kind of therapy is the high-dose brachytherapy Rhenium-SCT which makes use of the beta rays emitting property of rhenium-188. The radiation source is enclosed in a compound which is applied to a thin protection foile directly over the lesion. This way the radiation source can be applied to complex locations and minimize radiation to healthy tissue.[56]
After removal of the cancer, closure of the skin for patients with a decreased amount of skin laxity involves a split-thickness skin graft. A donor site is chosen and enough skin is removed so that the donor site can heal on its own. Only the epidermis and a partial amount of dermis is taken from the donor site which allows the donor site to heal. Skin can be harvested using either a mechanical dermatome or Humby knife.[57]
Electrodessication and curettage (EDC) can be done on selected squamous-cell carcinoma of the skin. In areas where cSCC is known to be non-aggressive, and where the patient is not immunosuppressed, EDC[clarification needed] can be performed with good to adequate cure rate.[58]
Treatment options for cSCC in situ (Bowen's disease) include photodynamic therapy with 5-aminolevulinic acid, cryotherapy, topical 5-fluorouracil or imiquimod, and excision. A meta-analysis showed evidence that PDT is more effective than cryotherapy and has better cosmetic outcomes. There is generally a lack of evidence comparing the effectiveness of all treatment options.[13]
High-risk squamous-cell carcinoma, as defined by that occurring around the eye, ear, or nose, is of large size, is poorly differentiated, and grows rapidly, requires more aggressive, multidisciplinary management.
Nodal spread:
- Surgical block dissection if palpable nodes or in cases of Marjolin's ulcers but the benefit of prophylactic block lymph node dissection with Marjolin's ulcers is not proven.
- Radiotherapy
- Adjuvant therapy may be considered in those with high-risk cSCC even in the absence of evidence for local metastasis. Imiquimod (Aldara) has been used with success for squamous-cell carcinoma in situ of the skin and the penis, but the morbidity and discomfort of the treatment is severe. An advantage is the cosmetic result: after treatment, the skin resembles normal skin without the usual scarring and morbidity associated with standard excision. Imiquimod is not FDA-approved for any squamous-cell carcinoma.
In general, squamous-cell carcinomas have a high risk of local recurrence, and up to 50% do recur.[59] Frequent skin exams with a dermatologist is recommended after treatment.
Prognosis
[edit]The long-term outcome of squamous-cell carcinoma is dependent upon several factors: the sub-type of the carcinoma, available treatments, location and severity, and various patient health-related variables (accompanying diseases, age, etc.). Generally, the long-term outcome is positive, with a metastasis rate of 1.9-5.2% and a mortality rate of 1.5-3.4%.[25][60][61]
When it does metastasize, the most commonly involved organs are the lungs, brain, bone and other skin locations.[62] Squamous-cell carcinoma occurring in immunosuppressed people (such as those with organ transplant, human immunodeficiency virus infection, or chronic lymphocytic leukemia) the risk of developing cSCC and having metastasis is much higher than the general population.[63]
One study found squamous-cell carcinoma of the penis had a much greater rate of mortality than some other forms of squamous-cell carcinoma, that is, about 23%,[64] although this relatively high mortality rate may be associated with possibly latent diagnosis of the disease due to patients avoiding genital exams until the symptoms are debilitating, or refusal to submit to a possibly scarring operation upon the genitalia.
Epidemiology
[edit]
The incidence of cutaneous squamous-cell carcinoma continues to rise around the world. This is theorized to be due to several factors; including an aging population, a greater incidence of those who are immunocompromised and the increasing use of tanning beds.[25]
A recent study estimated that there are between 180,000 and 400,000 cases of cSCC in the United States in 2013.[66] Risk factors for cSCC varies with age, gender, race, geography, and genetics. The incidence of cSCC increases with age and with those 75 years or older being at a 5-10 times increased risk of developing cSCC as compared with those who are younger than 55 years old.[25] Males are affected with cSCC at a ratio of 3:1 in comparison to females.[25] Those who have light skin, red or blonde hair and light colored eyes are also at increased risk.[25]
Squamous-cell carcinoma of the skin can be found on all areas of the body but is most common on frequently sun-exposed areas, such as the face, legs and arms.[67] Solid organ transplant recipients (heart, lung, liver, pancreas, among others) are also at a heightened risk of developing aggressive, high-risk cSCC. There are also a few rare congenital diseases predisposed to cutaneous malignancy. In certain geographic locations, exposure to arsenic in well water[68] or from industrial sources may significantly increase the risk of cSCC.[26]
Additional images
[edit]-
Biopsy-proven cutaneous squamous-cell carcinoma
-
Squamous-cell carcinoma of the dorsum of the hand
-
cSCC in situ (Bowen's disease)
-
cSCC of the right upper cheek; lesion outlined in blue with a dashed line prior to biopsy
-
Giant squamous cell carcinoma of the cheek
See also
[edit]References
[edit]- ^ a b Dunphy LM (2011). Primary Care: The Art and Science of Advanced Practice Nursing. F.A. Davis. p. 242. ISBN 9780803626478. Archived from the original on 2016-05-20.
- ^ a b c d e f g h i j Gandhi SA, Kampp J (November 2015). "Skin Cancer Epidemiology, Detection, and Management". The Medical Clinics of North America. 99 (6): 1323–1335. doi:10.1016/j.mcna.2015.06.002. PMID 26476255.
- ^ a b "Skin Cancer Treatment". National Cancer Institute. 21 June 2017. Archived from the original on 4 July 2017. Retrieved 2 July 2017.
- ^ a b c d Ferri FF (2016). Ferri's Clinical Advisor 2017 E-Book: 5 Books in 1. Elsevier Health Sciences. p. 1199. ISBN 9780323448383. Archived from the original on 29 August 2017. Retrieved 2 July 2017.
- ^ a b c d World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 5.14. ISBN 978-9283204299.
- ^ a b Jou PC, Feldman RJ, Tomecki KJ (June 2012). "UV protection and sunscreens: what to tell patients". Cleveland Clinic Journal of Medicine. 79 (6): 427–436. doi:10.3949/ccjm.79a.11110. PMID 22660875.
- ^ a b c "Skin Cancer Treatment". National Cancer Institute. 21 June 2017. Archived from the original on 4 July 2017. Retrieved 2 July 2017.
- ^ a b Vos, Theo; et al. (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- ^ a b Wang, Haidong; et al. (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/s0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- ^ "Skin Cancer Treatment (PDQ®)". NCI. 2013-10-25. Archived from the original on 5 July 2014. Retrieved 30 June 2014.
- ^ Cakir BÖ, Adamson P, Cingi C (November 2012). "Epidemiology and economic burden of nonmelanoma skin cancer". Facial Plastic Surgery Clinics of North America. 20 (4): 419–422. doi:10.1016/j.fsc.2012.07.004. PMID 23084294.
- ^ a b c d e f g h i j k l m n Yanofsky VR, Mercer SE, Phelps RG (2011). "Histopathological variants of cutaneous squamous cell carcinoma: a review". Journal of Skin Cancer. 2011: 210813. doi:10.1155/2011/210813. PMC 3018652. PMID 21234325.
 This article incorporates text available under the CC BY-SA 3.0 license.
This article incorporates text available under the CC BY-SA 3.0 license.
- ^ a b c Bath-Hextall FJ, Matin RN, Wilkinson D, Leonardi-Bee J (June 2013). "Interventions for cutaneous Bowen's disease". The Cochrane Database of Systematic Reviews. 2016 (6): CD007281. doi:10.1002/14651858.CD007281.pub2. PMC 6464151. PMID 23794286.
- ^ a b "Basal and Squamous Cell Skin Cancer Risk Factors". American Cancer Society.
- ^ Opel S, Ghali S (2016). "Skin Cancer for the Plastic Surgeon". Textbook of Plastic and Reconstructive Surgery (1 ed.). UCL Press. pp. 61–76. doi:10.2307/j.ctt1g69xq0.9. ISBN 978-1-910634-39-4. JSTOR j.ctt1g69xq0.9.
- ^ Gallagher RP, Lee TK, Bajdik CD, Borugian M (2010). "Ultraviolet radiation". Chronic Diseases in Canada. 29 (Suppl 1): 51–68. doi:10.24095/hpcdp.29.S1.04. PMID 21199599.
- ^ Lehmann AR, McGibbon D, Stefanini M (November 2011). "Xeroderma pigmentosum". Orphanet Journal of Rare Diseases. 6 (1): 70. doi:10.1186/1750-1172-6-70. PMC 3221642. PMID 22044607.
- ^ Bardhan A, Bruckner-Tuderman L, Chapple IL, Fine JD, Harper N, Has C, et al. (September 2020). "Epidermolysis bullosa". Nature Reviews. Disease Primers. 6 (1): 78. doi:10.1038/s41572-020-0210-0. PMID 32973163. S2CID 221861310.
- ^ "NCI Dictionary of Cancer Terms". National Cancer Institute. 2011-02-02. Archived from the original on 9 November 2016. Retrieved 9 November 2016.
- ^ a b Khandelwal AR, Kent B, Hillary S, Alam MM, Ma X, Gu X, et al. (October 2019). "Fibroblast growth factor receptor promotes progression of cutaneous squamous cell carcinoma". Molecular Carcinogenesis. 58 (10): 1715–1725. doi:10.1002/mc.23012. PMC 6721978. PMID 31254372.
- ^ Stratigos A, Garbe C, Lebbe C, Malvehy J, del Marmol V, Pehamberger H, et al. (September 2015). "Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline". European Journal of Cancer. 51 (14): 1989–2007. doi:10.1016/j.ejca.2015.06.110. PMID 26219687.
- ^ H, Carlos (2022-09-10). "How serious is a squamous-cell carcinoma?". CuradermBCC. Archived from the original on 2022-09-10. Retrieved 2022-09-10.
- ^ Thompson AK, Kelley BF, Prokop LJ, Murad MH, Baum CL (April 2016). "Risk Factors for Cutaneous Squamous Cell Carcinoma Recurrence, Metastasis, and Disease-Specific Death: A Systematic Review and Meta-analysis". JAMA Dermatology. 152 (4): 419–428. doi:10.1001/jamadermatol.2015.4994. PMC 4833641. PMID 26762219.
- ^ Schmults CD, Blitzblau R, Aasi SZ, Alam M, Andersen JS, Baumann BC, et al. (December 2021). "NCCN Guidelines® Insights: Squamous Cell Skin Cancer, Version 1.2022". Journal of the National Comprehensive Cancer Network. 19 (12): 1382–1394. doi:10.6004/jnccn.2021.0059. PMID 34902824. S2CID 245153567.
- ^ a b c d e f Wysong, Ashley (15 June 2023). "Squamous-Cell Carcinoma of the Skin". New England Journal of Medicine. 388 (24): 2262–2273. doi:10.1056/NEJMra2206348. PMID 37314707. S2CID 259154802.
- ^ a b "MD Consult - Important Notice". Archived from the original on 3 March 2016. Retrieved 15 March 2017.
- ^ "Squamous Cell Carcinoma". American Academy of Dermatology. 25 December 2010. Archived from the original on 25 December 2010. Retrieved 15 March 2017.
- ^ Decicco-Skinner KL, Trovato EL, Simmons JK, Lepage PK, Wiest JS (January 2011). "Loss of tumor progression locus 2 (tpl2) enhances tumorigenesis and inflammation in two-stage skin carcinogenesis". Oncogene. 30 (4): 389–397. doi:10.1038/onc.2010.447. PMC 3460638. PMID 20935675.
- ^ Dreyfuss I, Kamath P, Frech F, Hernandez L, Nouri K (January 2022). "Squamous cell carcinoma: 2021 updated review of treatment". Dermatologic Therapy. 35 (4): e15308. doi:10.1111/dth.15308. PMID 34997811. S2CID 245812900.
- ^ Wensveen CA, Bastiaens MT, Kielich CJ, Berkhout MJ, Westendorp RG, Vermeer BJ, Bouwes Bavinck JN (January 2001). "Relation between smoking and skin cancer". Journal of Clinical Oncology. 19 (1): 231–238. doi:10.1200/JCO.2001.19.1.231. PMID 11134217.
- ^ Wells JW, Goyal N, Najjar T, Monroe MM (28 June 2016). Meyers AD (ed.). "Cutaneous Squamous Cell Carcinoma: Practice Essentials, Background, Pathophysiology". Archived from the original on 6 April 2017. Retrieved 15 March 2017.
- ^ a b Marks Jr JG (2006). Lookingbill and Marks' principles of dermatology (4th ed.). Philadelphia, PA: Saunders Elsevier. p. 63. ISBN 978-1-4160-3185-7.
- ^ a b Freedberg IM, Fitzpatrick TB (2003). Fitzpatrick's Dermatology in General Medicine (6th ed.). New York: McGraw-Hill, Medical Pub. Division. ISBN 978-0-07-138076-8.
- ^ a b James WD, Berger TG, Elston DM (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. ISBN 0-7216-2921-0.
- ^ a b Rapini RP, Bolognia JL, Jorizzo JL (2007). Dermatology: 2-Volume Set. St. Louis: Mosby. p. 1050. ISBN 978-1-4160-2999-1.
- ^ a b c Zenilman JM, Shahmanesh M, eds. (2012). Sexually transmitted infections diagnosis, management, and treatment. Sudbury, Mass.: Jones & Bartlett Learning. p. 109. ISBN 9781449619848.
- ^ Wieland U, Jurk S, Weissenborn S, Krieg T, Pfister H, Ritzkowsky A (September 2000). "Erythroplasia of queyrat: coinfection with cutaneous carcinogenic human papillomavirus type 8 and genital papillomaviruses in a carcinoma in situ". The Journal of Investigative Dermatology. 115 (3): 396–401. doi:10.1046/j.1523-1747.2000.00069.x. PMID 10951274.
- ^ Shah IA, Sangi SA, Abbasi SA (1998). "Bowen's Disease". Pakistan Journal of Ophthalmology. 14 (1): 37–38.
- ^ a b Katsambas AD, Lotti TM, eds. (2003). European handbook of dermatological treatments (2nd ed.). Berlin [u.a.]: Springer. p. 169. ISBN 978-3-540-00878-1.
- ^ Chang, Darwin; Shain, A. Hunter (2021-07-16). "The landscape of driver mutations in cutaneous squamous cell carcinoma". npj Genomic Medicine. 6 (1): 61. doi:10.1038/s41525-021-00226-4. ISSN 2056-7944. PMC 8285521. PMID 34272401.
- ^ Parekh, Vishwas; Seykora, John T. (September 2017). "Cutaneous Squamous Cell Carcinoma". Clinics in Laboratory Medicine. 37 (3): 503–525. doi:10.1016/j.cll.2017.06.003. PMID 28802498.
- ^ Chang, Darwin; Shain, A. Hunter (2021-07-16). "The landscape of driver mutations in cutaneous squamous cell carcinoma". npj Genomic Medicine. 6 (1): 61. doi:10.1038/s41525-021-00226-4. ISSN 2056-7944. PMC 8285521. PMID 34272401.
- ^ Tessari G, Girolomoni G (October 2012). "Nonmelanoma skin cancer in solid organ transplant recipients: update on epidemiology, risk factors, and management". Dermatologic Surgery. 38 (10): 1622–1630. doi:10.1111/j.1524-4725.2012.02520.x. PMID 22805312. S2CID 40490951.
- ^ O'Reilly Zwald F, Brown M (August 2011). "Skin cancer in solid organ transplant recipients: advances in therapy and management: part I. Epidemiology of skin cancer in solid organ transplant recipients". Journal of the American Academy of Dermatology. 65 (2): 253–261. doi:10.1016/j.jaad.2010.11.062. PMID 21763561.
- ^ Chockalingam, Ramya; Downing, Christopher; Tyring, Stephen K. (June 2015). "Cutaneous Squamous Cell Carcinomas in Organ Transplant Recipients". Journal of Clinical Medicine. 4 (6): 1229–1239. doi:10.3390/jcm4061229. ISSN 2077-0383. PMC 4484997. PMID 26239556.
- ^ "Squamous Cell Carcinoma: What it Looks Like". SkinCancerNet. American Academy of Dermatology. 2008. Archived from the original on 2008-12-21.
- ^ Kuschal C, Thoms KM, Schubert S, Schäfer A, Boeckmann L, Schön MP, Emmert S (January 2012). "Skin cancer in organ transplant recipients: effects of immunosuppressive medications on DNA repair". Experimental Dermatology. 21 (1): 2–6. doi:10.1111/j.1600-0625.2011.01413.x. PMID 22151386. S2CID 25776283.
- ^ Khalyl-Mawad J (3 April 2021). "Pathology of Cutaneous Squamous Cell Carcinoma and Bowen Disease". Medscape. Updated: Jun 11, 2019
- ^ "Bowen's disease". National Health Service. 17 October 2017. Page last reviewed: 21 May 2019
- ^ Berkow R (1997). The Merck manual of medical information. Whitehouse Station, N.J: Merck Research Laboratories. p. 1057. ISBN 0911910875.
- ^ Gallagher RP, Lee TK, Bajdik CD, Borugian M (2010). "Ultraviolet radiation". Chronic Diseases in Canada. 29 (Suppl 1): 51–68. doi:10.24095/hpcdp.29.S1.04. PMID 21199599.
- ^ Sánchez G, Nova J, Rodriguez-Hernandez AE, Medina RD, Solorzano-Restrepo C, Gonzalez J, et al. (July 2016). "Sun protection for preventing basal cell and squamous cell skin cancers". The Cochrane Database of Systematic Reviews. 7 (9): CD011161. doi:10.1002/14651858.CD011161.pub2. PMC 6457780. PMID 27455163.
- ^ Lansbury L, Leonardi-Bee J, Perkins W, Goodacre T, Tweed JA, Bath-Hextall FJ (April 2010). "Interventions for non-metastatic squamous cell carcinoma of the skin". The Cochrane Database of Systematic Reviews. 2015 (4): CD007869. doi:10.1002/14651858.CD007869.pub2. PMC 10816961. PMID 20393962.
- ^ Gross KG, Steinman HK, Rapini RP, eds. (1999). Mohs Surgery, Fundamentals and Techniques. Mosby.
- ^ Krausz AE, Ji-Xu A, Smile T, Koyfman S, Schmults CD, Ruiz ES (May 2021). "A Systematic Review of Primary, Adjuvant, and Salvage Radiation Therapy for Cutaneous Squamous Cell Carcinoma". Dermatologic Surgery. 47 (5): 587–592. doi:10.1097/DSS.0000000000002965. PMID 33577212. S2CID 231899301.
- ^ Castellucci P, Savoia F, Farina A, Lima GM, Patrizi A, Baraldi C, et al. (May 2021). "High dose brachytherapy with non sealed 188Re (rhenium) resin in patients with non-melanoma skin cancers (NMSCs): single center preliminary results". European Journal of Nuclear Medicine and Molecular Imaging. 48 (5): 1511–1521. doi:10.1007/s00259-020-05088-z. PMC 8113182. PMID 33140131. S2CID 226231693.
- ^ [1], Hallock G. Squamous Cell Carcinoma Excision from Right Forearm with Split-Thickness Skin Graft from the Thigh. J Med Ins. 2020;2020(290.16) doi:https://jomi.com/article/290.16
- ^ Firnhaber JM (September 2020). "Basal Cell and Cutaneous Squamous Cell Carcinomas: Diagnosis and Treatment". American Family Physician. 102 (6): 339–346. PMID 32931212.
- ^ Gross MD, Cardash HS (March 1989). "Transferring anterior occlusal guidance to the articulator". The Journal of Prosthetic Dentistry. 61 (3): 282–285. doi:10.1016/0022-3913(89)90128-5. PMID 2921745.
- ^ Chollet A, Hohl D, Perrier P (April 2012). "[Risk of cutaneous squamous cell carcinomas: the role of clinical and pathological reports]". Revue Médicale Suisse. 8 (335): 743–746. doi:10.53738/REVMED.2012.8.335.0743. PMID 22545495.
- ^ Brantsch KD, Meisner C, Schönfisch B, Trilling B, Wehner-Caroli J, Röcken M, Breuninger H (August 2008). "Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study". The Lancet. Oncology. 9 (8): 713–720. doi:10.1016/S1470-2045(08)70178-5. PMID 18617440.
- ^ Major A, Anderson M (August 2017). "Not Just Skin Deep: Distant Metastases from Cutaneous Squamous Cell Carcinoma". The American Journal of Medicine. 130 (8): e327–e328. doi:10.1016/j.amjmed.2017.02.031. PMID 28344135.
- ^ Chapman JR, Webster AC, Wong G (July 2013). "Cancer in the transplant recipient". Cold Spring Harbor Perspectives in Medicine. 3 (7): a015677. doi:10.1101/cshperspect.a015677. PMC 3685882. PMID 23818517.
- ^ Bethune G, Campbell J, Rocker A, Bell D, Rendon R, Merrimen J (May 2012). "Clinical and pathologic factors of prognostic significance in penile squamous cell carcinoma in a North American population". Urology. 79 (5): 1092–1097. doi:10.1016/j.urology.2011.12.048. PMID 22386252.
- ^ "WHO Disease and injury country estimates". World Health Organization. 2009. Archived from the original on 2009-11-11. Retrieved Nov 11, 2009.
- ^ Karia PS, Han J, Schmults CD (June 2013). "Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012". Journal of the American Academy of Dermatology. 68 (6): 957–966. doi:10.1016/j.jaad.2012.11.037. PMID 23375456.
- ^ "Squamous Cell Carcinoma". T he Skin Cancer Foundation. 2018. Retrieved Dec 12, 2018.
- ^ Karagas MR, Gossai A, Pierce B, Ahsan H (March 2015). "Drinking Water Arsenic Contamination, Skin Lesions, and Malignancies: A Systematic Review of the Global Evidence". Current Environmental Health Reports. 2 (1): 52–68. doi:10.1007/s40572-014-0040-x. PMC 4522704. PMID 26231242.


![Squamous-cell carcinoma in situ, showing prominent dyskeratosis and aberrant mitoses at all levels of the epidermis, along with marked parakeratosis.[12]](http://upload.wikimedia.org/wikipedia/commons/thumb/b/ba/Micrograph_of_squamous_cell_carcinoma_in_situ_-_100x.jpg/367px-Micrograph_of_squamous_cell_carcinoma_in_situ_-_100x.jpg)
![cSCC in situ, high magnification, demonstrating an intact basement membrane.[12]](http://upload.wikimedia.org/wikipedia/commons/thumb/0/01/Micrograph_of_squamous_cell_carcinoma_in_situ_-_400x.jpg/299px-Micrograph_of_squamous_cell_carcinoma_in_situ_-_400x.jpg)





![Superficially invasive cutaneous squamous-cell carcinoma. These lesions often do not show the marked pleomorphism and atypical nuclei of cSCC in situ, but manifest early keratinocyte invasion of the dermis.[12]](http://upload.wikimedia.org/wikipedia/commons/thumb/f/f8/Micrograph_of_invasive_squamous_cell_carcinoma_-_150x.jpg/305px-Micrograph_of_invasive_squamous_cell_carcinoma_-_150x.jpg)
![High magnification demonstrates the pleomorphism of the invading keratinocytes[12]](http://upload.wikimedia.org/wikipedia/commons/thumb/2/26/Micrograph_of_invasive_squamous_cell_carcinoma_-_200x.jpg/340px-Micrograph_of_invasive_squamous_cell_carcinoma_-_200x.jpg)

![Well-differentiated (yet invasive) cSCC, showing prominent keratinization. It may form pearl-like structures where dermal nests of keratinocytes attempt to mature in a layered fashion. Well-differentiated cSCC has slightly enlarged hyperchromatic nuclei with abundant amounts of cytoplasm. Intercellular bridges will frequently be visible.[12]](http://upload.wikimedia.org/wikipedia/commons/thumb/3/3c/Micrograph_of_well-differentiated_and_invasive_squamous-cell_carcinoma.jpg/306px-Micrograph_of_well-differentiated_and_invasive_squamous-cell_carcinoma.jpg)
![Moderately differentiated lesions of invasive cSCC show much less organization and maturation with significantly less keratin formation.[12]](http://upload.wikimedia.org/wikipedia/commons/thumb/f/f2/Micrograph_of_moderately_differentiated_and_invasive_squamous-cell_carcinoma.jpg/310px-Micrograph_of_moderately_differentiated_and_invasive_squamous-cell_carcinoma.jpg)
![Poorly differentiated, where attempts at keratinization are often no longer evident. This is a clear-cell squamous-cell carcinoma. The dysplastic cells infiltrated cords through the dermis. Poorly differentiated cSCC has greatly enlarged pleomorphic nuclei showing a high degree of atypia and frequent mitoses.[12]](http://upload.wikimedia.org/wikipedia/commons/thumb/6/61/Micrograph_of_clear-cell_squamous-cell_carcinoma.jpg/296px-Micrograph_of_clear-cell_squamous-cell_carcinoma.jpg)