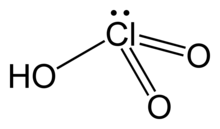
Chloric acid
| |

| |
| Names | |
|---|---|
| Other names
Chloric(V) acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.303 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 2626 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| HClO3 | |
| Molar mass | 84.45914 g mol−1 |
| Appearance | colourless solution |
| Density | 1 g/mL, solution (approximate) |
| >40 g/100 ml (20 °C) | |
| Acidity (pKa) | −2.7[1] |
| Conjugate base | Chlorate |
| Structure | |
| pyramidal | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Oxidant, Corrosive |
| GHS labelling: | |
 
| |
| Danger | |
| H271, H314 | |
| P210, P220, P221, P260, P264, P280, P283, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P306+P360, P310, P321, P363, P370+P378, P371+P380+P375, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
bromic acid iodic acid |
Other cations
|
ammonium chlorate sodium chlorate potassium chlorate |
Related compounds
|
hydrochloric acid hypochlorous acid chlorous acid perchloric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chloric acid, HClO3, is an oxoacid of chlorine, and the formal precursor of chlorate salts. It is a strong acid (pKa ≈ −2.7) and an oxidizing agent.
Properties
[edit]Chloric acid is thermodynamically unstable with respect to disproportionation.
Chloric acid is stable in cold aqueous solution up to a concentration of approximately 30%, and solution of up to 40% can be prepared by careful evaporation under reduced pressure. Above these concentrations, chloric acid solutions decompose to give a variety of products, for example:
- 8 HClO3 → 4 HClO4 + 2 H2O + 2 Cl2 + 3 O2
- 3 HClO3 → HClO4 + H2O + 2 ClO2
Hazards
[edit]Chloric acid is a powerful oxidizing agent. Most organics and flammables will deflagrate on contact.
Production
[edit]It can be prepared by the reaction of sulfuric acid with barium chlorate, the insoluble barium sulfate being removed by precipitation:
- Ba(ClO3)2 + H2SO4 → 2 HClO3 + BaSO4
Another method is the heating of hypochlorous acid, producing chloric acid and hydrogen chloride:
- 3 HClO → HClO3 + 2 HCl
See also
[edit]References
[edit]- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- R. Bruce King, ed. (1994). "Chloric acid". Encyclopedia of Inorganic Chemistry. Vol. 2. Chichester: Wiley. p. 658. ISBN 0-471-93620-0.

